A 30 Gram Animal That Moves Slowly Along The Ocean Bottom Would Best Attain Oxygen By Which Means?
Dissolved Oxygen
What is Dissolved Oxygen?
Dissolved oxygen refers to the level of free, not-compound oxygen present in water or other liquids. It is an important parameter in assessing water quality because of its influence on the organisms living within a body of h2o. In limnology (the study of lakes), dissolved oxygen is an essential gene 2nd only to h2o itself ¹. A dissolved oxygen level that is besides high or too low can impairment aquatic life and touch on h2o quality.
Non-compound oxygen, or gratuitous oxygen (O2), is oxygen that is not bonded to any other element. Dissolved oxygen is the presence of these free O2 molecules within water.The bonded oxygen molecule in h2o (H2o) is in a compound and does not count toward dissolved oxygen levels. I tin can imagine that free oxygen molecules dissolve in water much the way salt or sugar does when it is stirred ².
Dissolved Oxygen and Aquatic Life
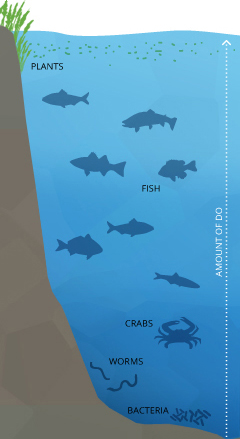
Dissolved oxygen is necessary to many forms of life including fish, invertebrates, bacteria and plants. These organisms utilise oxygen in respiration, like to organisms on land. Fish and crustaceans obtain oxygen for respiration through their gills, while plant life and phytoplankton require dissolved oxygen for respiration when in that location is no lite for photosynthesis iv. The corporeality of dissolved oxygen needed varies from animal to creature. Lesser feeders, crabs, oysters and worms need minimal amounts of oxygen (1-six mg/Fifty), while shallow water fish need higher levels (iv-15 mg/L)⁵.
Microbes such every bit bacteria and fungi also crave dissolved oxygen. These organisms utilise Exercise to decompose organic material at the bottom of a bounding main. Microbial decomposition is an of import contributor to food recycling. Still, if there is an excess of decomposable organic material (from dying algae and other organisms), in a body of h2o with exceptional or no turnover (likewise known as stratification), the oxygen at lower h2o levels will become used upwards quicker ⁶.
Where Does Practice Come up From?
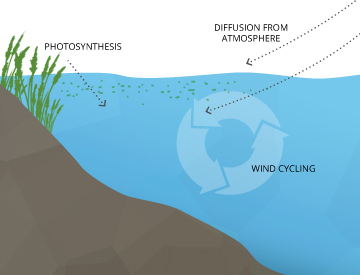
Dissolved oxygen enters water through the air or every bit a establish byproduct. From the air, oxygen tin slowly lengthened across the water's surface from the surrounding atmosphere, or be mixed in quickly through aeration, whether natural or homo-made 7. The aeration of h2o can exist caused past wind (creating waves), rapids, waterfalls, ground water belch or other forms of running water. Human being-fabricated causes of aeration vary from an aquarium air pump to a mitt-turned waterwheel to a large dam.
Dissolved oxygen is also produced as a waste product of photosynthesis from phytoplankton, algae, seaweed and other aquatic plants 8.
Dissolved Oxygen from Photosynthesis
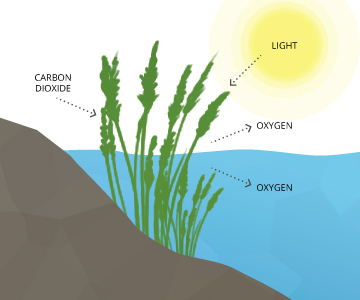
While nigh photosynthesis takes identify at the surface (by shallow water plants and algae), a big portion of the process takes identify underwater (by seaweed, sub-surface algae and phytoplankton). Light tin can penetrate h2o, though the depth that it tin can achieve varies due to dissolved solids and other light-scattering elements nowadays in the water. Depth also affects the wavelengths bachelor to plants, with red being absorbed quickly and blue light being visible by 100 g. In clear water, there is no longer enough light for photosynthesis to occur beyond 200 m, and aquatic plants no longer grow. In turbid water, this photic (light-penetrating) zone is oftentimes much shallower.
Regardless of wavelengths bachelor, the cycle doesn't change ⁹. In addition to the needed light, CO2 is readily captivated by water (it's about 200 times more soluble than oxygen) and the oxygen produced as a byproduct remains dissolved in h2o¹⁰. The basic reaction of aquatic photosynthesis remains:
CO2 + H2o → (CH2O) + O2
As aquatic photosynthesis is light-dependent, the dissolved oxygen produced will acme during daylight hours and decline at night ⁸.
Dissolved Oxygen Saturation
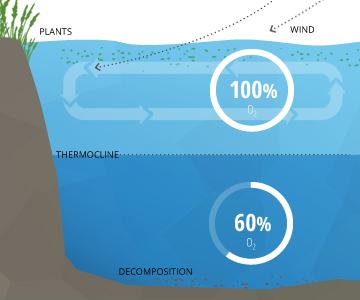
In a stable body of h2o with no stratification, dissolved oxygen will remain at 100% air saturation. 100% air saturation means that the h2o is holding as many dissolved gas molecules every bit it can in equilibrium. At equilibrium, the percent of each gas in the water would be equivalent to the percentage of that gas in the atmosphere – i.east. its partial pressure ¹³. The water will slowly absorb oxygen and other gasses from the atmosphere until it reaches equilibrium at consummate saturation 10. This procedure is sped up by wind-driven waves and other sources of aeration ³.
In deeper waters, DO can remain below 100% due to the respiration of aquatic organisms and microbial decomposition. These deeper levels of water often do not achieve 100% air saturation equilibrium considering they are not shallow enough to exist affected by the waves and photosynthesis at the surface ³. This water is beneath an invisible boundary chosen the thermocline (the depth at which water temperature begins to decline)¹¹.
What Affects Oxygen Solubility?
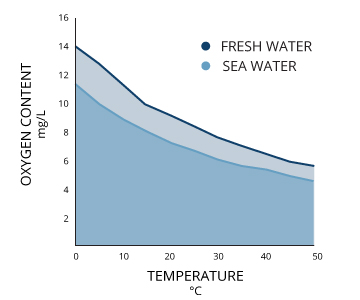
Ii bodies of water that are both 100% air-saturated exercise non necessarily have the aforementioned concentration of dissolved oxygen. The actual amount of dissolved oxygen (in mg/50) volition vary depending on temperature, pressure and salinity ¹.
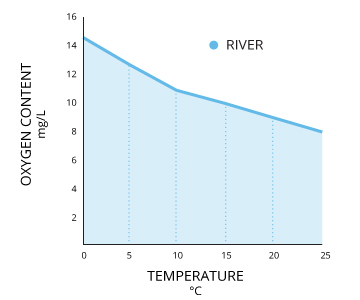
Starting time, the solubility of oxygen decreases as temperature increases ¹. This means that warmer surface water requires less dissolved oxygen to achieve 100% air saturation than does deeper, cooler water. For example, at bounding main level (1 atm or 760 mmHg) and 4°C (39°F), 100% air-saturated water would concur ten.92 mg/L of dissolved oxygen. ³ Only if the temperature were raised to room temperature, 21°C (70°F), there would only be 8.68 mg/L Practise at 100% air saturation ³.
Second dissolved oxygen decreases exponentially as salt levels increase ¹. That is why, at the same pressure and temperature, saltwater holds nearly 20% less dissolved oxygen than freshwater ³.
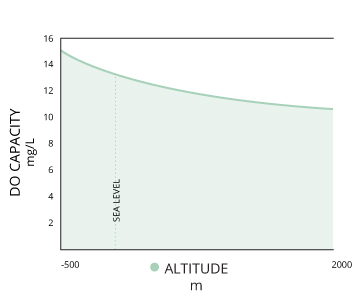
Third, dissolved oxygen will increase as pressure increases ¹. This is true of both atmospheric and hydrostatic pressures. Water at lower altitudes can hold more than dissolved oxygen than h2o at college altitudes. This relationship as well explains the potential for "supersaturation" of waters below the thermocline – at greater hydrostatic pressures, water can hold more dissolved oxygen without information technology escaping ¹. Gas saturation decreases past 10% per meter increment in depth due to hydrostatic pressure ¹². This means that if the concentration of dissolved oxygen is at 100% air saturation at the surface, it would only be at lxx% air saturation three meters beneath the surface.
In summary, colder, deeper fresh waters have the capability to hold higher concentrations of dissolved oxygen, but due to microbial decomposition, lack of atmospheric contact for diffusion and the absence of photosynthesis, actual Practise levels are often far below 100% saturation ¹⁰. Warm, shallow saltwater reaches 100% air saturation at a lower concentration, but can often accomplish levels over 100% due to photosynthesis and aeration. Shallow waters also remain closer to 100% saturation due to atmospheric contact and constant diffusion ¹⁰.
If there is a significant occurrence of photosynthesis or a rapid temperature change, the h2o tin can achieve DO levels over 100% air saturation. At these levels, the dissolved oxygen will dissipate into the surrounding water and air until information technology levels out at 100% ³.
How Tin can Water be More 100% Saturated?
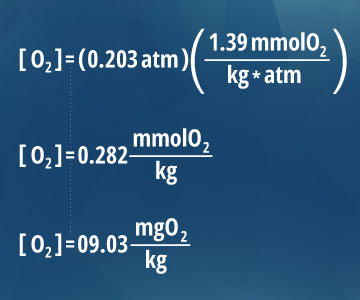
100% air saturation is the equilibrium betoken for gases in water. This is because gas molecules diffuse betwixt the temper and the water'due south surface. Co-ordinate to Henry's Law, the dissolved oxygen content of water is proportional to the percent of oxygen (fractional pressure) in the air above it thirteen. As oxygen in the atmosphere is about twenty.3%, the partial pressure of oxygen at bounding main level (one atm) is 0.203 atm. Thus the amount of dissolved oxygen at 100% saturation at body of water level at 20° C is ix.03 mg/L ¹⁰.
The equation shows that h2o will remain at 100% air saturation at equilibrium. However, there are several factors that tin affect this. Aquatic respiration and decomposition lower DO concentrations, while rapid aeration and photosynthesis can contribute to supersaturation. During the process of photosynthesis, oxygen is produced equally a waste matter product. This adds to the dissolved oxygen concentration in the water, potentially bringing it above 100% saturation ¹⁴. In addition, the equalization of water is a ho-hum process (except in highly agitated or aerated situations). This means that dissolved oxygen levels can easily be more 100% air saturation during the day in photosynthetically agile bodies of water ¹⁴.
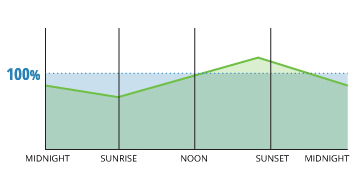

Supersaturation caused by rapid aeration is frequently seen beside hydro-power dams and large waterfalls ¹². Unlike small rapids and waves, the water flowing over a dam or waterfall traps and carries air with it, which is then plunged into the water. At greater depths and thus greater hydrostatic pressures, this entrained air is forced into solution, potentially raising saturation levels over 100% ¹².
Rapid temperature changes tin also create DO readings greater than 100% ¹⁴. As water temperature rises, oxygen solubility decreases. On a cool summer night, a lake's temperature might be 60° F. At 100% air saturation, this lake'south dissolved oxygen levels would be at 9.66 mg/L. When the sun rises and warms up the lake to lxx° F, 100% air saturation should equate to 8.68 mg/L DO ³. Merely if there is no wind to move the equilibration along, the lake will however comprise that initial 9.66 mg/L Exercise, an air saturation of 111%.
Typical Dissolved Oxygen Levels
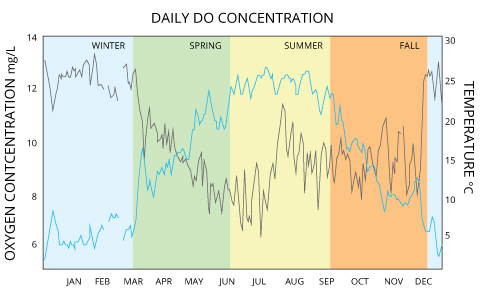
Dissolved oxygen concentrations are constantly afflicted past diffusion and aeration, photosynthesis, respiration and decomposition. While water equilibrates toward 100% air saturation, dissolved oxygen levels will also fluctuate with temperature, salinity and pressure level changes ³. As such, dissolved oxygen levels can range from less than 1 mg/50 to more than 20 mg/L depending on how all of these factors interact. In freshwater systems such as lakes, rivers and streams, dissolved oxygen concentrations volition vary by season, location and h2o depth.
Freshwater Fluctuations: Example 1
In the Pompton River in New Jersey, mean dissolved oxygen concentrations range from 12-13 mg/L in winter and drop to 6-9 mg/50 in the summertime ⁸. That same river shows daily fluctuations of upwardly to 3 mg/L due to photosynthesis production ⁸.
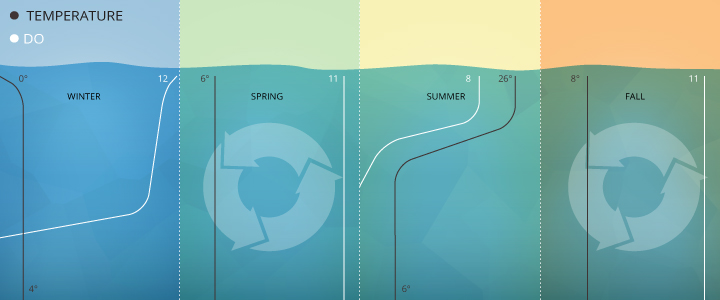
Freshwater Fluctuations: Instance two
Studies at Crooked Lake in Indiana show dissolved oxygen concentrations vary by season and depth from 12 mg/L (surface, winter) to 0 mg/L (32 thousand depth, late summer), with full lake turnovers in spring and autumn equalizing Practise levels around xi mg/L for all depths ¹.
Rivers and streams tend to stay near or slightly above 100% air saturation due to relatively large surface areas, aeration from rapids, and groundwater discharge, which means that their dissolved oxygen concentrations volition depend on the water temperature ¹. While groundwater usually has low DO levels, groundwater-fed streams can hold more oxygen due to the influx of colder water and the mixing it causes ¹⁵. Standard Methods for the Examination of Water and Wastewater defines dissolved oxygen in streams as the sum of photosynthetic byproducts, respiration, re-aeration, accrual from groundwater inflow and surface runoff ¹³.
Saltwater holds less oxygen than freshwater, and then oceanic Do concentrations tend to be lower than those of freshwater. In the ocean, surface water mean almanac Practice concentrations range from 9 mg/L well-nigh the poles downwardly to iv mg/L near the equator with lower DO levels at farther depths. There are lower dissolved oxygen concentrations virtually the equator considering salinity is higher ¹⁶.
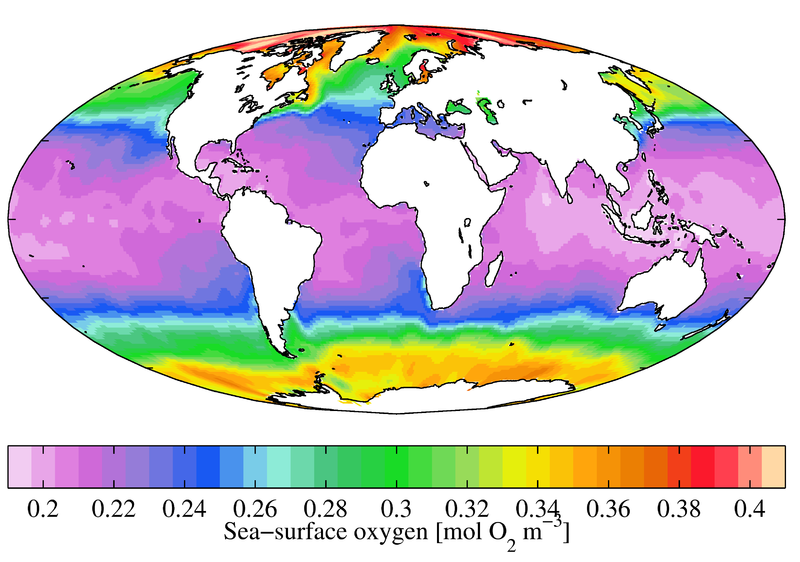
Some states have Water Quality Standard Acts, requiring minimum concentrations of dissolved oxygen; in Michigan, these minimums are 7 mg/L for common cold-water fisheries and v mg/50 for warm-water fish 17; in Colorado, "Class 1 Cold Water Aquatic Life" needs vi mg/Fifty, and "Class 1 Warm H2o Aquatic Life" requires DO levels of at least 5 mg/50 15. In order to mimic ideal environmental systems, freshwater tanks ideally need around 8 mg/L Do for optimum growth and marine tank requirements range from 6-7 mg/50 DO based on the salinity level ¹⁸. In other words, dissolved oxygen should exist near 100% air saturation.
Examples of Freshwater Organisms and Dissolved Oxygen Requirements

Coldwater fish similar trout and salmon are most affected by low dissolved oxygen levels 19. The mean DO level for developed salmonids is half-dozen.v mg/L, and the minimum is four mg/L ¹². These fish mostly attempt to avoid areas where dissolved oxygen is less than 5 mg/L and will brainstorm to die if exposed to Exercise levels less than 3 mg/Fifty for more than than a couple days ¹⁹. For salmon and trout eggs, dissolved oxygen levels below xi mg/L will delay their hatching, and beneath 8 mg/L will impair their growth and lower their survival rates. ¹⁹ When dissolved oxygen falls below 6 mg/50 (considered normal for most other fish), the vast majority of trout and salmon eggs volition dice. ¹⁹
Bluegill, Largemouth Bass, White Perch, and Yellow Perch are considered warmwater fish and depend on dissolved oxygen levels in a higher place 5 mg/L21. They will avoid areas where Practice levels are beneath iii mg/L, simply by and large do not brainstorm to endure fatalities due to oxygen depletion until levels fall below ii mg/L 22. The mean DO levels should remain near 5.5 mg/Fifty for optimum growth and survival ¹².
Walleye too prefer levels over 5 mg/50, though they can survive at 2 mg/L DO levels for a brusque fourth dimension.²⁴ Muskie need levels over 3 mg/L for both adults and eggs ²⁵. Carp are hardier, and while they tin can enjoy dissolved oxygen levels higher up 5 mg/50, they easily tolerate levels beneath ii mg/L and can survive at levels below 1 mg/L ²⁶.
The freshwater fish most tolerant to DO levels include fathead minnows and northern expressway. Northern pike can survive at dissolved oxygen concentrations as low as 0.1 mg/L for several days, and at one.5 mg/Fifty for an space amount of fourth dimension ²⁷. Fathead minnows can survive at 1 mg/L for an extended period with only minimal effects on reproduction and growth.
Equally for bottom-dwelling microbes, Practise changes don't carp them much. If all the oxygen at their h2o level gets used up, bacteria will beginning using nitrate to decompose organic thing, a procedure known equally denitrification. If all of the nitrogen is spent, they will begin reducing sulfate ¹⁷. If organic affair accumulates faster than information technology decomposes, sediment at the bottom of a lake just becomes enriched by the organic material. ²⁸.
Examples of Saltwater Organisms and Dissolved Oxygen Requirements
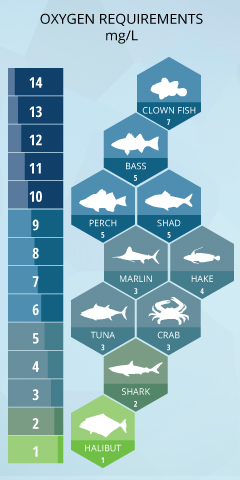
Saltwater fish and organisms have a higher tolerance for depression dissolved oxygen concentrations every bit saltwater has a lower 100% air saturation than freshwater. In full general, dissolved oxygen levels are about twenty% less in seawater than in freshwater ³.
This does not mean that saltwater fish tin live without dissolved oxygen completely. Striped bass, white perch and American shad need Do levels over 5 mg/50 to grow and thrive ⁵. The carmine hake is besides extremely sensitive to dissolved oxygen levels, abandoning its preferred habitat near the seafloor if concentrations fall below 4.2 mg/L ²⁹.
The dissolved oxygen requirements of open-ocean and deep-ocean fish are a bit harder to track, but there have been some studies in the area. Billfish swim in areas with a minimum of iii.5 mg/Fifty DO, while marlins and sailfish volition dive to depths with DO concentrations of ane.v mg/Fifty ³⁰. Also, white sharks are besides limited in dive depths due to dissolved oxygen levels (in a higher place ane.5 mg/L), though many other sharks have been found in areas of depression Exercise ³³. Tracked swordfish evidence a preference for shallow h2o during the twenty-four hours, basking in oxygenated water (7.7 mg/L) later on diving to depths with concentrations around 2.5 mg/L ³⁴. Albacore tuna live in mid-ocean levels, and require a minimum of ii.5 mg/50 ³⁵, while halibut tin can maintain a minimum DO tolerance threshold of 1 mg/L ³⁶.
Many tropical saltwater fish, including clown fish, angel fish and groupers require higher levels of DO, such as those surrounding coral reefs. Coral reefs are plant in the euphotic zone (where low-cal penetrates the h2o – normally non deeper than 70 k). College dissolved oxygen concentrations are generally institute effectually coral reefs due to photosynthesis and aeration from eddies and breaking waves ³⁷. These DO levels tin fluctuate from 4-fifteen mg/L, though they normally remain effectually 5-8 mg/L, cycling betwixt day photosynthesis production and night plant respiration ³⁸. In terms of air saturation, this means that dissolved oxygen near coral reefs tin hands range from xl-200% ³⁹.
Crustaceans such as crabs and lobsters are benthic (bottom-domicile) organisms, just even so require minimum levels of dissolved oxygen. Depending on the species, minimum Do requirements tin range from iv mg/L to ane mg/L ¹³. Despite being bottom dwellers, mussels, oysters and clams as well require a minimum of ane-two mg/L of dissolved oxygen 29, which is why they are establish in shallower, littoral waters that receive oxygen from the atmosphere and photosynthetic sources.
Consequences of Unusual Do Levels
If dissolved oxygen concentrations drop below a certain level, fish mortality rates volition rising. Sensitive freshwater fish like salmon can't fifty-fifty reproduce at levels below 6 mg/Fifty ¹⁹. In the sea, coastal fish begin to avert areas where DO is beneath 3.7 mg/Fifty, with specific species abandoning an area completely when levels autumn beneath 3.five mg/Fifty ²⁹. Below two.0 mg/L, invertebrates also leave and beneath 1 mg/L even benthic organisms prove reduced growth and survival rates ²⁹.
Fish impale / Winterkill
A fishkill occurs when a large number of fish in an area of water die off. It can be species-based or a h2o-broad bloodshed. Fish kills can occur for a number of reasons, but low dissolved oxygen is oft a gene. A winterkill is a fish kill caused past prolonged reduction in dissolved oxygen due to ice or snow comprehend on a lake or swimming ²⁰.

When a body of water is overproductive, the oxygen in the water may get used up faster than it can be replenished. This occurs when a trunk of water is overstocked with organisms or if in that location is a large algal bloom die-off.
Fish kills are more common in eutrophic lakes: lakes with loftier concentrations of nutrients (particularly phosphorus and nitrogen) ⁴¹. High levels of nutrients fuel algae blooms, which can initially boost dissolved oxygen levels. But more than algae ways more than plant respiration, cartoon on Practice, and when the algae dice, bacterial decomposition spikes, using upward about or all of the dissolved oxygen bachelor. This creates an anoxic, or oxygen-depleted, surroundings where fish and other organisms cannot survive. Such nutrient levels can occur naturally, but are more than ofttimes caused by pollution from fertilizer runoff or poorly treated wastewater ⁴¹.
Winterkills occur when respiration from fish, plants and other organisms is greater than the oxygen production by photosynthesis ¹. They occur when the water is covered by ice, and then cannot receive oxygen by improvidence from the atmosphere. If the ice is so covered past snow, photosynthesis besides cannot occur, and the algae will depend entirely on respiration or die off. In these situations, fish, plants and decomposition are all using up the dissolved oxygen, and information technology cannot exist replenished, resulting in a winter fish kill. The shallower the water, and the more productive (loftier levels of organisms) the water, the greater the likelihood of a winterkill ²⁰.
Gas Bubble Disease

Simply every bit low dissolved oxygen can crusade problems, and so too can high concentrations. Supersaturated water tin cause gas bubble disease in fish and invertebrates ¹². Significant death rates occur when dissolved oxygen remains above 115%-120% air saturation for a menstruation of fourth dimension. Total bloodshed occurs in immature salmon and trout in nether three days at 120% dissolved oxygen saturation ¹². Invertebrates, while likewise affected past gas bubble disease, tin usually tolerate higher levels of supersaturation than fish ¹².
Extended periods of supersaturation can occur in highly aerated waters, often near hydropower dams and waterfalls, or due to excessive photosynthetic activeness. Algae blooms tin crusade air saturations of over 100% due to large amounts of oxygen equally a photosynthetic byproduct. This is oftentimes coupled with higher water temperatures, which also affects saturation. ¹² At higher temperatures, h2o becomes 100% saturated at lower concentrations, and then higher dissolved oxygen concentrations mean fifty-fifty higher air saturation levels.
Dead Zones
A dead zone is an expanse of h2o with little to no dissolved oxygen present. They are so named because aquatic organisms cannot survive there. Dead zones often occur nigh heavy man populations, such equally estuaries and littoral areas off the Gulf of United mexican states, the North Sea, the Baltic Sea, and the East Red china Ocean. They tin occur in large lakes and rivers every bit well, merely are more well known in the oceanic context.
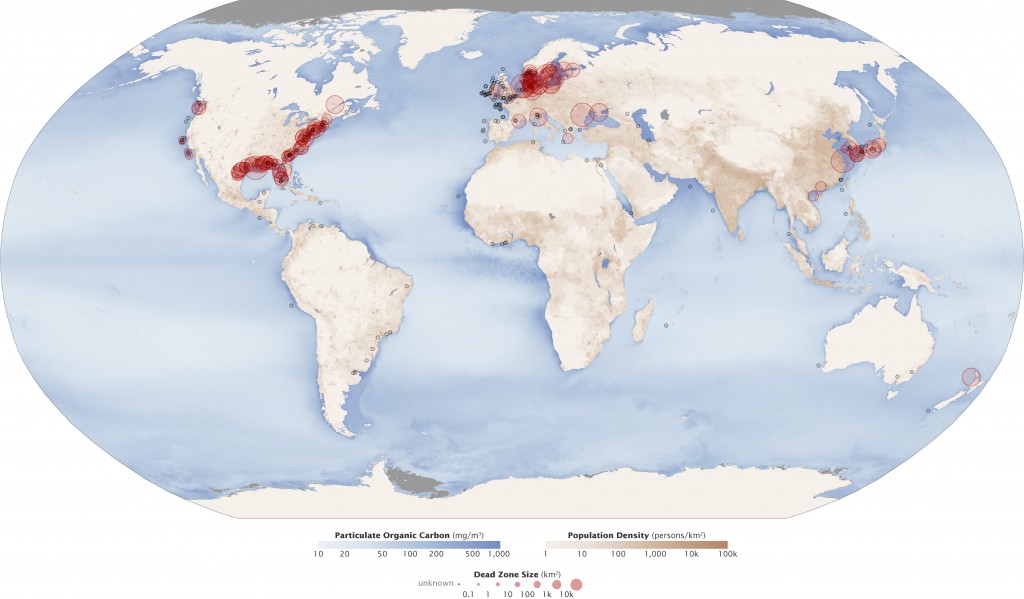
These zones are usually a result of a fertilizer-fueled algae and phytoplankton growth smash. When the algae and phytoplankton dice, the microbes at the seafloor employ up the oxygen decomposing the organic thing ³¹. These anoxic conditions are usually stratified, occurring just in the lower layers of the water. While some fish and other organisms tin escape, shellfish, young fish and eggs usually die ³².
Naturally occurring hypoxic (low oxygen) conditions are not considered dead zones. The local aquatic life (including benthic organisms) have adjusted to the recurring low-oxygen conditions, so the adverse effects of a dead zone (mass fish kills, sudden disappearance of aquatic organisms, and growth/development problems in fish and invertebrates) practise not occur ³¹.
Such naturally occurring zones frequently occur in deep lake basins and lower ocean levels due to water column stratification.
Dissolved Oxygen and Water Column Stratification
Stratification separates a torso of water into layers. This layering can be based on temperature or dissolved substances (namely table salt and oxygen) with both factors oft playing a role. The stratification of water has been normally studied in lakes, though it also occurs in the body of water. Information technology can also occur in rivers if pools are deep plenty and in estuaries where there is a significant division between freshwater and saltwater sources.
Lake Stratification
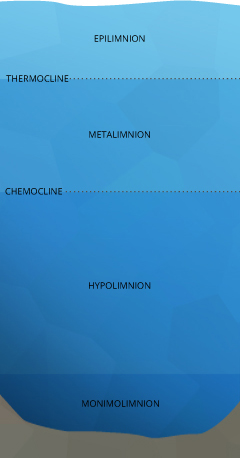
The uppermost layer of a lake, known as the epilimnion, is exposed to solar radiations and contact with the atmosphere, keeping information technology warmer. The depth of the epilimnion is dependent on the temperature exchange, commonly determined by h2o clarity and depth of mixing (unremarkably initiated by wind) ¹¹. Inside this upper layer, algae and phytoplankton engage in photosynthesis. Between the contact with the air, potential for aeration and the byproducts of photosynthesis, dissolved oxygen in the epilimnion remains near 100% saturation. The exact levels of DO vary depending on the temperature of the water, the amount of photosynthesis occurring and the quantity of dissolved oxygen used for respiration past aquatic life.
Below the epilimnion is the metalimnion, a transitional layer that fluctuates in thickness and temperature. The boundary between the epilimnion and metalimnion is called the thermocline – the point at which water temperature begins to steadily drop off ¹¹. Here, two different outcomes can occur. If light can penetrate beyond the thermocline and photosynthesis occurs in this strata, the metalimnion tin achieve an oxygen maximum ¹¹. This means that the dissolved oxygen level will be higher in the metalimnion than in the epilimnion. But in eutrophic or nutrient-rich lakes, the respiration of organisms tin can deplete dissolved oxygen levels, creating a metalimnetic oxygen minimum ⁴².
The side by side layer is the hypolimnion. If the hypolimnion is deep enough to never mix with the upper layers, information technology is known as the monimolimnion. The hypolimnion is separated from the upper layers by the chemocline or halocline. These clines mark the boundary between oxic and anoxic water and salinity gradients, respectively. ¹¹. While lab conditions would conclude that at colder temperatures and higher pressures water can hold more dissolved oxygen, this is not ever the effect. In the hypolimnion, leaner and fungi use dissolved oxygen to decompose organic material ⁶. This organic textile comes from dead algae and other organisms that sink to the bottom. The dissolved oxygen used in decomposition is non replaced – there is no atmospheric contact, aeration or photosynthesis to restore Do levels in the hypolimnion ¹¹. Thus the process of decomposition "uses up" all of the oxygen within this layer.
If the lake in question is a holomictic "mixing" lake, all the layers mix at least once per yr (usually jump and autumn) when lake strata temperatures align. This turnover redistributes dissolved oxygen throughout all the layers and the process begins again.
Ocean Stratification
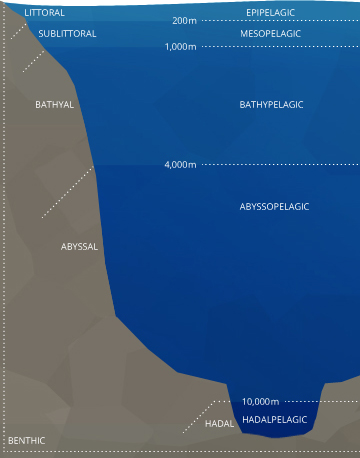
Stratification in the ocean is both horizontal and vertical. The littoral, or coastal area is most affected by estuaries and other inflow sources.⁴⁴ It tends to exist shallow and tidal with fluctuating dissolved oxygen levels. The sublittoral, also known as the neritic or demersal zone, is considered a littoral zone too. In this zone, dissolved oxygen concentrations may vary just they practise not fluctuate as much every bit they do in the littoral zone.
This is the zone where many coral reefs grow, and Practise levels remain near 100% air saturation due to eddies, breaking waves and photosynthesis 45. This zone is also where most oceanic benthic (lesser-dwelling) organisms exist. Oceanic benthic fish do not live at the greatest depths of the sea. They dwell at the seafloor most to coasts and oceanic shelves while remaining in the upper levels of the bounding main.
Across the demersal zone are the bathyal, abyssal and hadal plains, which are adequately similar in terms of consistently low DO.
In the open sea, in that location are five major vertical strata: epipelagic, mesopelagic, bathypelagic, abyssopelagic, and hadalpelagic ⁴⁴. The exact definitions and depths are subjective, but the following information is mostly agreed upon. The epipelagic is also known as the surface layer or photic zone (where light penetrates). This is the layer with the highest levels of dissolved oxygen due to wave activity and photosynthesis. The epipelagic generally reaches to 200 m and is bordered by a drove of clines.
These clines tin can overlap or be at separate depths. Much like in a lake, the thermocline divides oceanic strata by temperature. The halocline divides by salinity levels and the pycnocline divides strata by density ¹⁶. Each of these clines can affect the corporeality of dissolved oxygen the ocean strata can hold.
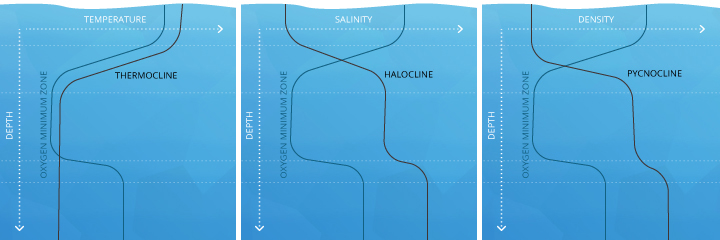
The mesopelagic, significant "twilight" zone, stretches from 200-1000 m. Depending on water clarity, some light may filter through, merely at that place is not enough for photosynthesis to occur ⁴⁴. Within this strata, the oxygen minimum zone (OMZ) can occur. The OMZ develops because organisms apply the oxygen for respiration, but it is too deep to exist replenished past photosynthetic oxygen byproducts or aeration from waves. This zone tends to exist effectually a depth of 500 m ⁴⁵. The mesopelagic zone is bordered by chemoclines (clines based on chemistry levels, east.thousand. oxygen and salinity) on both sides, reflecting unlike dissolved oxygen and salinity levels between the strata.
Below the mesopelagic is the aphotic zone(s). These strata have lower dissolved oxygen levels than the surface water because photosynthesis does not occur but can take college levels than the OMZ because less respiration occurs.
The bathypelagic, "midnight" zone exists betwixt 1000-4000 m, and many creatures can still live here. The bottom layer of the sea is the abyssopelagic, which exists below 4000 chiliad. The hadopelagic is the proper noun for the zone of deep sea trenches that open below the abyssal evidently, such as the Mariana Trench ⁴⁴.
Estuary Stratification
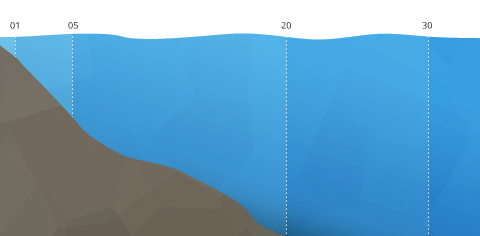
Estuary stratifications are based on salinity distributions. Because saltwater holds less dissolved oxygen than freshwater, this can touch on aquatic organism distribution. The stronger the river flow, the higher the oxygen concentrations. This stratification tin can be horizontal, with DO levels dropping from inland to open bounding main, or vertical, with the fresh, oxygenated river water floating over the depression Do seawater ⁴⁶. When the stratification is clearly defined, a pycnocline divides the fresher h2o from the salt water, contributing to separate dissolved oxygen concentrations in each strata.
Dissolved Oxygen Units and Reporting

Dissolved oxygen is usually reported in milligrams per liter (mg/L) or as a per centum of air saturation. However, some studies volition report DO in parts per million (ppm) or in micromoles (umol). 1 mg/L is equal to 1 ppm. The relationship betwixt mg/Fifty and % air saturation has been discussed in a higher place, and varies with temperature, pressure and salinity of the water. One micromole of oxygen is equal to 0.022391 milligrams, and this unit is normally used in oceanic studies ⁴⁷. Thus 100 umol/L O2 is equal to 2.2 mg/L O2.
Calculating DO from % Air Saturation
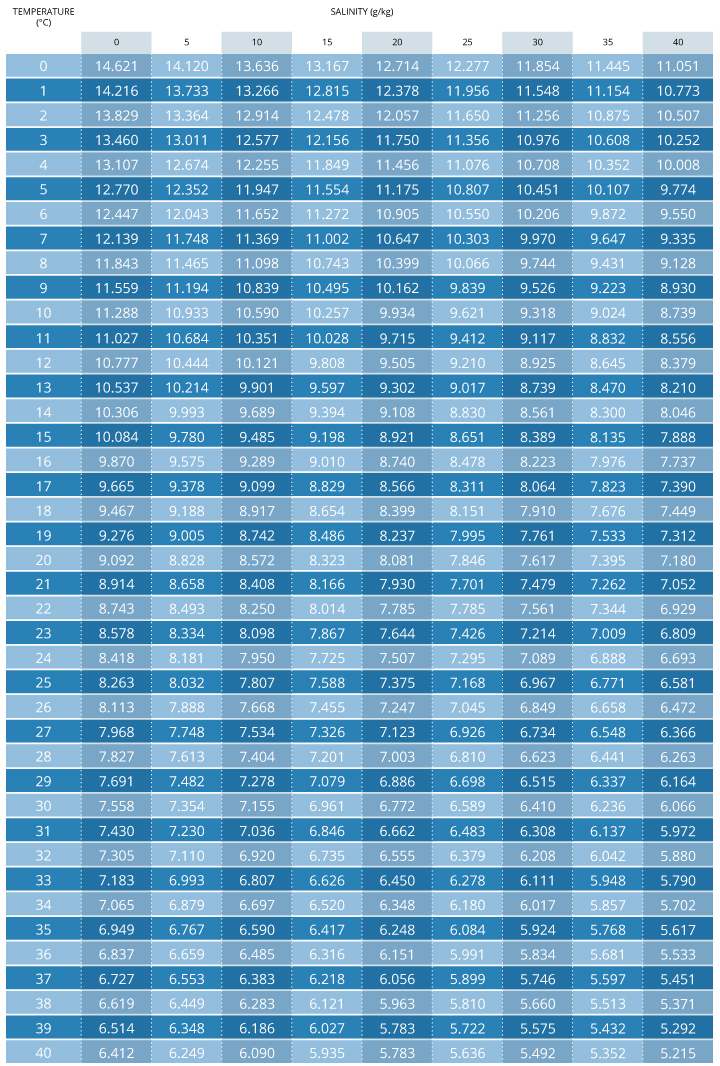
To calculate dissolved oxygen concentrations from air saturation, it is necessary to know the temperature and salinity of the sample. Barometric pressure has already been accounted for as the partial pressure of oxygen contributes to the per centum air saturation vii. Salinity and temperature can so be used in Henry's Law to calculate what the Practice concentration would be at 100% air saturation 10. However, it is easier to use an oxygen solubility chart. These charts show the dissolved oxygen concentration at 100% air saturation at varying temperatures, and salinities. This value can and so be multiplied by the measured percent air saturation to calculate the dissolved oxygen concentration seven.
O2 mg/Fifty = (Measured % Practise)*(Practise value from chart at temperature and salinity)
Case:
70% Exercise measured
35 ppt salinity
15°C
.70 * 8.135 = 5.69 mg/L DO
Cite This Piece of work
Fondriest Ecology, Inc. "Dissolved Oxygen." Fundamentals of Environmental Measurements. nineteen Nov. 2013. Web. < https://world wide web.fondriest.com/environmental-measurements/parameters/h2o-quality/dissolved-oxygen/ >.
Additional Data
- Dissolved Oxygen Measurement Methods
- Dissolved Oxygen Sensors
- Dissolved Oxygen Meters
- Applications
- References
Source: https://www.fondriest.com/environmental-measurements/parameters/water-quality/dissolved-oxygen/
Posted by: coleywhely1977.blogspot.com

0 Response to "A 30 Gram Animal That Moves Slowly Along The Ocean Bottom Would Best Attain Oxygen By Which Means?"
Post a Comment